Apremilast, an inhibitor of phosphodiesterase 4, is used to treat adults with active psoriasis or arthritis psoriatic. It is sold under the common name Otezla and acts as a selective inhibitor of the enzyme PDE4. A patient suffering from plague psoriasis or adults with Behçet’s disease can be given this medication. Apremilast is an orally active drug used to reduce TNF-α and matrix metalloproteinase-3 production in patients with bowel disease.
Enquire Now
What is Apremilast?
Product Description
Molar Mass: 460.5
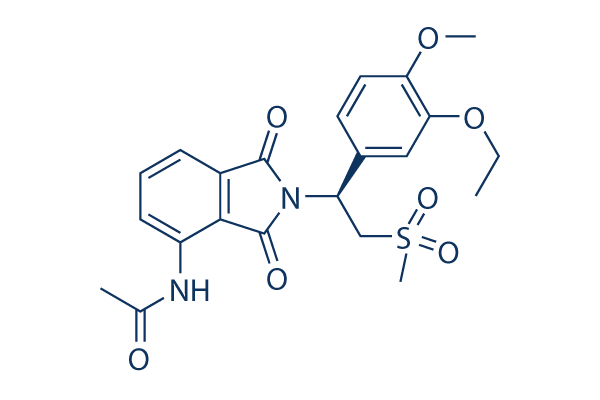
SMILES: CCOC1=C(OC)C=CC(=C1)C(CS(C)(=O)=O)N2C(=O)C3=C(C2=O)C(NC(C)=O)=CC=C3
InChIKey: IMOZEMNVLZVGJZ-UHFFFAOYSA-N
ALogP: 2.43
CAS Number: 608141-41-9
Chemical Formula: C22H24N2O7S
Form: Oral tablet
Bioavailability: 73%
Solubility: DMF: 20 mg/ml, DMF:PBS(pH 7.2)(1:1): 0.5 mg/ml, DMSO: 10 mg/ml
Storage: -20°C
Assay: NLT 99.0%
Suitable for: Adults
IUPAC Name: N-[2-[(1S)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl]-2,3-dihydro-1,3-dioxo-1H-isoindol-4-yl]-acetamide
Approved Indications: 2
As per the clinical trials, there is one approved indication of the Apremilast drug:
- Arthritis, Psoriatic
- Psoriasis
Experimental Indications: 40
As per clinical trials 1-4, the drug can be used in the following categories:
- Acne Conglobata (Phase 2)
- Acne Vulgaris (Phase 2)
- Alcoholism (Phase 2)
- Alopecia (Phase 4)
- Arthritis, Psoriatic ()
- Arthritis, Rheumatoid (Phase 2)
- Behcet Syndrome (Phase 3)
- Cardiovascular Diseases (Phase 4)
- Colitis, Ulcerative (Phase 2)
- COVID-19 (Phase 3)
- Dermatitis, Allergic Contact (Phase 2)
- Dermatitis, Atopic (Phase 2)
- Dermatology (Phase 2)
- Dermatomyositis (Phase 2)
- Digital Dermatitis (Phase 4)
- Eczema (Phase 2)
- Erythema (Phase 4)
- Genital Diseases, Female (Phase 2)
- Gout (Phase 2)
- Healthy Volunteers (Phase 1)
- Hidradenitis Suppurativa (Phase 2)
- Lichen Planus (Phase 2)
- Lichen Planus, Oral (Phase 2)
- Lupus Erythematosus, Discoid (Phase 1/Phase 2)
- Osteoarthritis (Phase 2)
- Parapsoriasis (Phase 4)
- Pharmacokinetics (Phase 1)
- Prostatitis (Phase 2)
- Prurigo (Phase 2)
- Pruritus (Phase 2)
- Psoriasis ()
- Rosacea (Phase 2)
- Sarcoidosis (Phase 3)
- Spondylitis, Ankylosing (Phase 3)
- Stomatitis, Aphthous (Phase 3)
- Substance Withdrawal Syndrome (Phase 3)
- Uveitis (Phase 1/Phase 2)
- Vitiligo (Phase 2)
- Vulva (Phase 2)
- Vulvodynia (Phase 2)
Clinical Trials:
One hundred and ten (110) trials took place for Apremilast in four phases. 94 organizations participated in the trials.
Side Effects:
Major side effects observed after the consumption of Apremilast are:
- Diarrhea
- Nausea
- Stomach Pain
- Vomiting
- Headache
Product CAS No
608141-41-9
Therapeutic Action
Apremilast is type of arthritis (psoriatic arthritis). Apremilast is also used to treat a certain type of skin condition (moderate to severe plaque psoriasis). Apremilast belongs to a class of drugs known as phosphodiesterase 4 (PDE4) inhibitors.
Conclusion
Apremilast is an orally active drug used to treat patients with psoriasis or arthritis psoriatic conditions. It has a molar mass of 460.5 g/mol and is sold under the brand name Otezla. Apremilast blocks the formation of TNF-α and matrix metalloproteinase-3 production in patients with inflammatory bowel conditions. Apart from psoriasis, the drug also has an experimental indication for the diseases like alopecia, vitiligo, rosacea, Behcet Syndrome, etc. Some common side effects in patients taking Apremilast drug are diarrhea, nausea, headache, and vomiting.

