Baricitinib is a Janus kinase inhibitor used to treat moderate to severe rheumatoid arthritis. It is sold under the brand name Olumiant and is non-biologic, meaning it is not made from living cells. Besides rheumatoid arthritis, Baricitinib is also used extensively in treating COVID 19. Baricitinib is used along with methotrexate to treat adults who have inadequate responses to one or more TNF antagonist therapies.
Enquire Now
What is Baricitinib?
Product Description
Molar Mass: 371.417
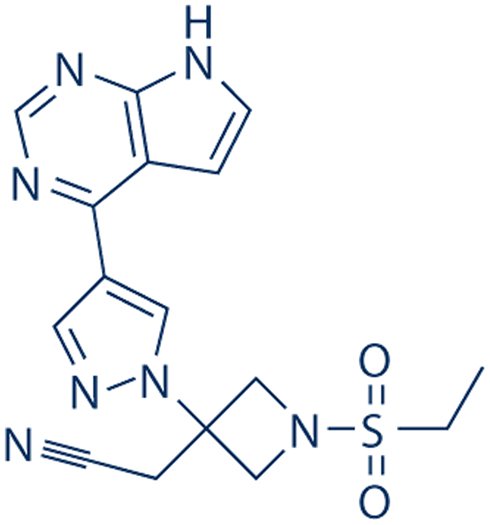
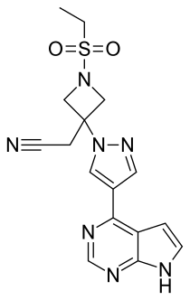
SMILES: CCS(=O)(=O)N1CC(CC#N)(C1)N2C=C(C=N2)C3=C4C=CNC4=NC=N3
InChIKey: XUZMWHLSFXCVMG-UHFFFAOYSA-N
ALogP: 1.1
CAS Number: 1187594-09-7
Chemical Formula: C16H17N7O2S
Form: Oral Tablet
Bioavailability: 79%
Solubility: DMF: 50 mg/ml, DMF:PBS(pH 7.2)(1:1): 0.5 mg/ml, DMSO: 30 mg/ml
Storage: -20°C
Suitable for: Adults
IUPAC Name:
2-[1-Ethylsulfonyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrazol-1-yl]azetidin-3-yl]acetonitrile
Approved Indications: 1
As per the clinical trials, there is one approved indication of the Baricitinib drug:
- Arthritis, Rheumatoid
Experimental Indications: 40
As per clinical trials 1-4, the drug can be used in the following categories:
- Alopecia Areata (Phase 3)
- Arthritis, Juvenile (Phase 3)
- Arthritis, Rheumatoid (Phase 4)
- Bone Density (Phase 3)
- Communicable Diseases, Emerging (Phase 2/Phase 3)
- Coronavirus Infections (Phase 3)
- COVID-19 (Phase 4)
- Dermatitis, Allergic Contact (Early Phase 1)
- Dermatitis, Atopic (Phase 3)
- Dermatomyositis (Phase 3)
- Diabetes Mellitus, Type 1 (Phase 2)
- Diabetic Nephropathies (Phase 2)
- Finger Joint (Phase 3)
- Giant Cell Arteritis (Phase 2)
- Gout (Phase 3)
- Healthy Volunteers (Phase 1)
- Hepatic Insufficiency (Phase 1)
- Hereditary Autoinflammatory Diseases (Phase 3)
- Inflammation (Phase 1)
- Lipodystrophy (Phase 3)
- Livedoid Vasculopathy (Phase 3)
- Liver Cirrhosis, Biliary (Phase 2)
- Liver Diseases (Phase 1)
- Lupus Erythematosus, Systemic (Phase 3)
- Myalgia (Phase 2)
- Myositis (Phase 2)
- Pharmacology (Phase 3)
- Pneumonia (Phase 2/Phase 3)
- Pneumonia, Viral (Phase 3)
- Psoriasis (Phase 2)
- Pyoderma (Phase 2)
- Pyoderma Gangrenosum (Phase 2)
- Sjogren’s Syndrome (Phase 2)
- Skin Diseases (Phase 2)
- Skin Diseases, Papulosquamous (Phase 2)
- Skin Ulcer (Phase 2)
- Uveitis (Phase 3)
- Vitiligo (Phase 2)
- Wound Healing (Phase 2)
Clinical Trials:
A total of 91 trials were held for Baricitinib in four phases. 54 organizations participated in these trials.
Side Effects:
Major side effects observed after the consumption of Baricitinib are:
- Sore Throat
- Headache
- Cough
- Chills
Product CAS No
1187594-09-7
Therapeutic Action
Baricitinib is used to treat rheumatoid arthritis.Baricitinib is being studied for use in combination with another drug (remdesivir) to treat coronavirus disease (COVID-19).The most common serious infections include pneumonia, shingles, and urinary tract infections.
Conclusion
Baricitinib is a Janus kinase inhibitor drug used to treat rheumatic arthritis and COVID 19 issues in adults. People with moderately to severely active rheumatoid arthritis can take this drug orally once a day. Patients requiring non-invasive or invasive ventilation and supplemental oxygen are also prescribed the same medication. Baricitinib has a molar mass of 371.47 g/mol. Some common experimental indications of Baricitinib are liver disease, weak bone intensity, coronavirus, alopecia areata, etc.

