Valsartan/Sacubitril is a combination of two powerful drugs used to reduce the risk of cardiovascular death and hospitalization in chronic heart failure. This combination is sold under the common name Entresto and contains Neprilysin Inhibitor Sacubitril and the Angiotensin Receptor Blocker Valsartan.
Enquire Now
What is Valsartna/Sacubitril?
Product Description:
Molar Mass: 1916.018 g·mol−1
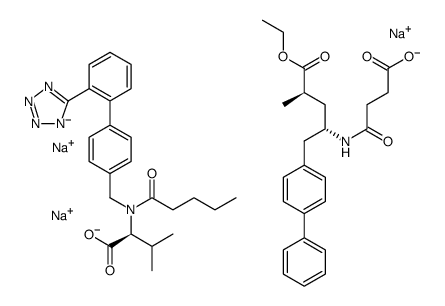
SMILES: CCCCC(=O)N(CC1=CC=C(C=C1)C2=CC=CC=C2C3=NN=N[N-]3)C(C(C)C)C(=O)[O-].CCCCC(=O)N(CC1=CC=C(C=C1)C2=CC=CC=C2C3=NN=N[N-]3)C(C(C)C)C(=O)[O-].CCOC(=O)C(C)CC(CC1=CC=C(C=C1)C2=CC=CC=C2)NC(=O)CCC(=O)[O-].CCOC(=O)C(C)CC(CC1=CC=C(C=C1)C2=CC=CC=C2)NC(=O)CCC(=O)[O-].O.O.O.O.O.[Na+].
[Na+].[Na+].[Na+].[Na+].[Na+]
InChI: 1S/2C24H29N5O3.2C24H29NO5.6Na.5H2O/c2*1-4-5-10-21(30)29(22(16(2)3)24(31)32)15-17-11-13-18(14-12-17)19-8-6-7-9-20(19)23-25-27-28-26-23;2*1-3-30-24(29)17(2)15-21(25-22(26)13-14-23(27)28)16-18-9-11-20(12-10-18)19-7-5-4-6-8-19;;;;;;;;;;;/h2*6-9,11-14,16,22H,4-5,10,15H2,1-3H3,(H2,25,26,27,28,31,32);2*4-12,17,21H,3,13-16H2,1-2H3,(H,25,26)(H,27,28);;;;;;;5*1H2/q;;;;6*+1;;;;;/p-6/t2*22-;2*17-,21+;;;;;;;;;;;/m0011………../s1
Key: ZASXKEGREHRXDL-CAWNUZPDSA-H
ALogP: 3.84
CAS Number: 936623-90-4
Therapeutic Category: angiotensin receptor blockers (ARBs)
Assay: 99%
Storage: 3 years -20°C powder, 2 years -80°C in solvent
Suitable for: Adults
Chemical Formula: C96H120N12Na6O21
Application: Patients with heart failure or a reduced LVEF (Left Ventricular Ejection Fraction) are normally given this fixed-dose combination drug.
IUPAC Name:
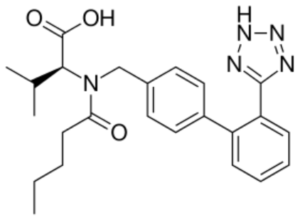
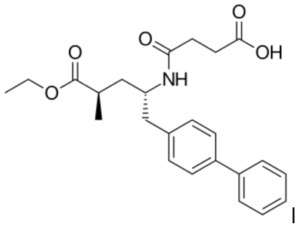
Sacubitril: 4-{[(2S,4R)-1-(4-Biphenylyl)-5-ethoxy-4-methyl-5-oxo-2-pentanyl]amino}-4-oxobutanoic acid. Valsartan’s IUPAC name is: (S)-3-methyl-2-(N-{[2′-(2H-1,2,3,4-tetrazol-5-yl)biphenyl-4-yl]methyl}pentanamido)butanoic acid.
Approved Indications (1)
As per the clinical trials, there is one approved indication of Sacubitril:
- Cardiovascular Diseases
Experimental Indications (52)
As per clinical trials 1-4, Valsartan/Sacubitril can be used in the following categories:
- Arrhythmias, Cardiac (Phase 2)
- Blood Pressure (Phase 4)
- Breast Neoplasms (Phase 2)
- Chagas Cardiomyopathy (Phase 3)
- Chagas Disease (Phase 4)
- Diabetes Mellitus, Type 2 (Phase 4)
- Endomyocardial Fibrosis (Phase 2)
- Erectile Dysfunction (Phase 3)
- Essential Hypertension (Phase 3)
- Heart Failure, Diastolic (Phase 4)
- Heart Failure, Systolic (Phase 4)
- Hemodialysis, Home (Phase 4)
- HIV Infections (Phase 2)
- Hypertension (Phase 4)
- Hypertension, Pulmonary (Phase 3)
- Hypertrophy, Left Ventricular (Phase 4)
- Hypotension (Phase 4)
- Insulin Resistance (Phase 2)
- Kidney Diseases (Phase 2)
- Metabolic Diseases (Phase 2)
- Mitral Valve Insufficiency (Phase 4)
- Myositis (Phase 2)
- Natriuretic Peptides (Phase 2/Phase 3)
- Obesity (Phase 4)
- Peripheral Arterial Disease (Phase 2)
- Prediabetic State (Phase 4)
- Shock, Cardiogenic (Phase 4)
- Thyroid Neoplasms (Phase 1)
- Ventricular Dysfunction, Left (Phase 4)
Clinical Trials
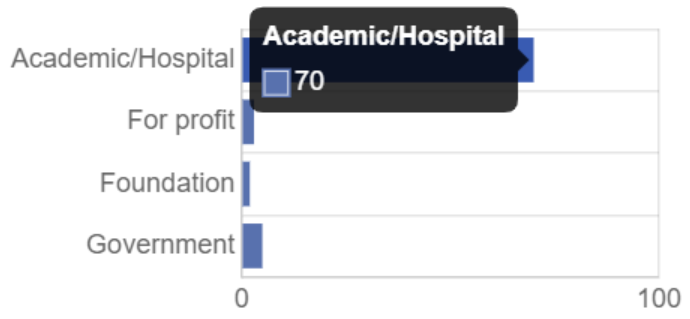
111 trials were held in a total of four phases. 80 organizations took part in the medical trials of Valsartan/Sacubitril.
Side Effects
The common side effects that may be seen in the patients taking Valsartan/Sacubitril are as follows:
- Hyperkalaemia ( high potassium levels in the blood)
- Hypotension ( low blood pressure)
- Reduced Kidney Function
- A Persistent Dry Cough
Angioedema can also be seen in some patients, which is not a common but rather a serious effect of Valsartan/Sacubitril.
Conclusion
Valsartan/Sacubitril is a powerful fixed-dose combination drug commonly sold under Entresto. This medicine is antihypertensive and used to treat cardiovascular and high blood pressure diseases. Valsartan /Sacubitril is taken in the tablet form orally with common dosages of 24mg/26mg, 49mg/51mg, and 97mg/103mg. You might experience a dry cough or improper kidney function due to the intake of this drug.

