Ledipasvir, also sold by Harvoni, is an antiviral drug used in fixed-dose combination with Sofosbuvir to treat HCV. The medicine eliminates the replication of NS5A protein which is the main cause of Hepatitis C.
Enquire Now
What is Ledipasvir?
Product Description:
Molar Mass: 889.018 g·mol−1
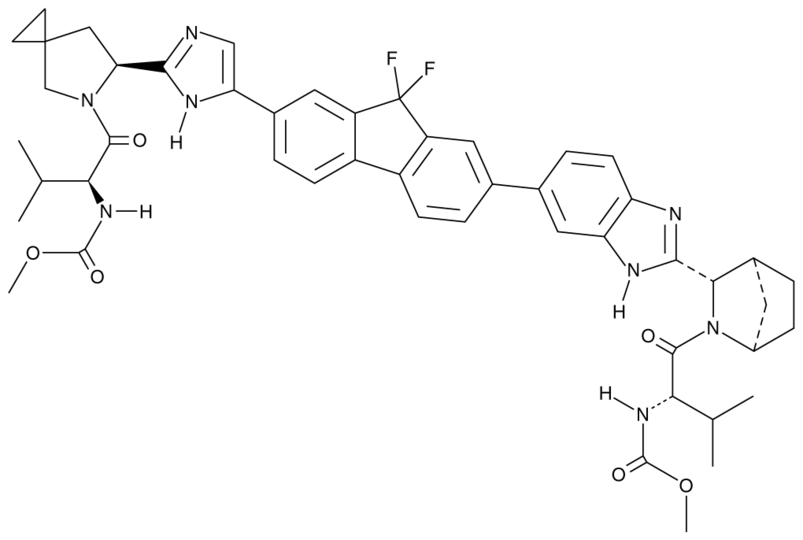
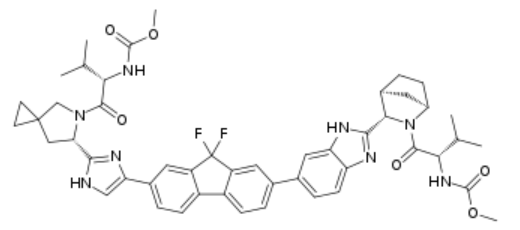
SMILES: COC(=O)N[C@@H](C(C)C)C(=O)N1CC2(CC2)C[C@H]1C3=NC=C(N3)C4=CC=C5C(=C4)C(F)(F)C6=C5C=CC(=C6)C7=CC8=C(C=C7)N=C(N8)[C@@H]9[C@H]%10CC[C@H](C%10)N9C(=O)[C@@H](NC(=O)OC)C(C)C
InChIKey: VRTWBAAJJOHBQU-KMWAZVGDSA-N
ALogP: 8.61
CAS Number: 1256388-51-8
Chemical Formula: C49H54F2N8O6
Therapeutic Category: AntiHCV
Form: Tablet
Assay: NLT 99.0%
Storage: -20C Freezer / 4C Coldroom
Shipping: Under room temperature
IUPAC Name:
Methyl N-[(2S)-1-[(6S)-6-[5-[9,9-Difluoro-7-[2-[(1S,2S,4R)-3-[(2S)-2-(methoxycarbonylamino)-3-methylbutanoyl]-3-azabicyclo[2.2.1]heptan-2-yl]-3H-benzimidazol-5-yl]fluoren-2-yl]-1H-imidazol-2-yl]-5-azaspiro[2.4]heptan-5-yl]-3-methyl-1-oxobutan-2-yl]carbamate. It’s chemical structure is shown below:
Approved Indication (2)
- Hepatitis C
- Hepatitis C, Chronic
Experimental Indication (27)
- beta-Thalassemia (Phase 4)
- Carcinoma, Hepatocellular (Phase 4)
- COVID-19 (Phase 4)
- Cryoglobulinemia (Phase 2/Phase 3)
- Fibrosis (Phase 4)
- Gaucher Disease (Phase 4)
- Healthy Volunteers (Phase 1)
- Heart Failure (Phase 4)
- Hematologic Neoplasms (Phase 1/Phase 2)
- Hematopoietic Stem Cell Transplantation (Phase 4)
- Hepacivirus (Phase 3)
- Hepatitis (Phase 1)
- Hepatitis B (Phase 3)
- Hepatitis C ()
- Hepatitis C, Chronic ()
- HIV (Phase 4)
- HIV Infections (Phase 4)
- Insulin Resistance (Phase 4)
- Liver Cirrhosis (Phase 3)
- Liver Diseases (Phase 4)
- Lung Diseases, Interstitial (Phase 4)
- Lymphoma (Phase 2)
- Lymphoma, B-Cell (Phase 2)
- Porphyria Cutanea Tarda (Phase 2)
- Pregnancy (Phase 1)
- Pulmonary Disease, Chronic Obstructive (Phase 4)
- Substance Abuse, Intravenous (Phase 3)
Clinical Trial (112)
A total of 112 trials took place for Ledispavir in four phases. 124 organizations took part in these trials.
Side Effects
Some of the common side effects that are seen with patients taking Ledipasvir are:
- Fatigue
- Headache
- Diarrhea
- Nausea
- Sleeping Disorders
Conclusion
Ledipasvir is an antiviral drug generally used with Sofosbuvir to treat HCV and other liver infections. This antiviral drug belongs to the nucleotide polymerase inhibitors medication class and has a molar mass of 889.018 g·mol−1. You can take this medicine orally with the recommended dosage of 90 mg per day. Headache, fatigue, and nausea are common side effects experienced by patients taking Ledipasvir.

