Osimertinib belongs to the kinase inhibitor medication class and is used to treat non-small-cell lung carcinomas. This drug is sold under the brand name Tagrisso which inhibits the proliferation of mutant EGFR-containing non-small cell lung cancer (NSCLC) cells. It is generally in the crystalline solid form and should be stored at room temperature. The stability of Osimertinib is ≥ 2 years.
Enquire Now
What is Osimertinib?
Product Description
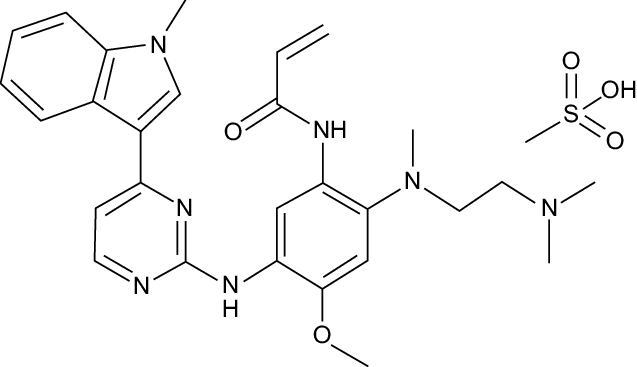
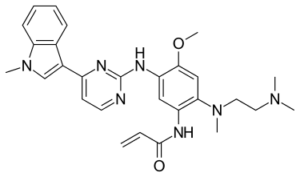
Molar Mass: 499.62
SMILES: C=CC(=O)Nc1cc(Nc2nccc(-c3cn(C)c4ccccc34)n2)c(OC)cc1N(C)CCN(C)C
InChIKey: DUYJMQONPNNFPI-UHFFFAOYSA-N
ALogP: 4.51
CAS Number: 1421373-65-0
Chemical Formula: C28H33N7O2
Form: Tablet
Protein Binding: High
Solubility: DMF: 25 mg/ml, DMSO: 15 mg/ml, Ethanol: 10 mg/ml
Storage: -20°C
Suitable for: Adults
IUPAC Name: N-(2-{[2-(dimethylamino)ethyl](methyl)amino}-4-methoxy-5-{[4-(1-methyl-1H-indol-3-yl)pyrimidin-2-yl]amino}phenyl)prop-2-enamide
Approved Indications: 1
As per the clinical trials, there is one major approved indication of the Osimertinib drug:
- Carcinoma, Non-Small-Cell Lung
Experimental Indications: 52
As per clinical trials 1-4, the drug can be used in the following categories:
- Adenocarcinoma (Phase 2)
- Adenocarcinoma of Lung (Phase 2)
- Antineoplastic Agents (Phase 1/Phase 2)
- Bile Duct Neoplasms (Phase 2)
- Brain Neoplasms (Phase 3)
- Breast Neoplasms (Phase 2)
- Bronchial Neoplasms (Phase 1/Phase 2)
- Carcinoma (Phase 2)
- Carcinoma, Bronchogenic (Phase 1/Phase 2)
- Carcinoma, Non-Small-Cell Lung ()
- Carcinoma, Squamous Cell (Phase 2)
- Cognition Disorders (Phase 2)
- Colonic Neoplasms (Phase 2)
- Colorectal Neoplasms (Phase 2)
- Disease Progression (Phase 2)
- Drugs, Investigational (Phase 3)
- Endometrial Neoplasms (Phase 2)
- Epidermal Growth Factor (Phase 2)
- Esophageal Neoplasms (Phase 2)
- Glioblastoma (Phase 2)
- Glioma (Phase 2)
- Healthy Volunteers (Phase 1)
- Kidney Neoplasms (Phase 2)
- Liver Neoplasms (Phase 2)
- Lung Diseases (Phase 1/Phase 2)
- Lung Neoplasms (Phase 3)
- Lymphoma (Phase 2)
- Melanoma (Phase 2)
- Multiple Myeloma (Phase 2)
- Mutation (Phase 3)
- Neoplasm Metastasis (Phase 2)
- Neoplasms ()
- Neoplasms by Histologic Type (Phase 1/Phase 2)
- Neoplasms by Site (Phase 1/Phase 2)
- Neoplasms, Nerve Tissue (Phase 1/Phase 2)
- Ovarian Neoplasms (Phase 2)
- Pancreatic Neoplasms (Phase 2)
- Positron Emission Tomography Computed Tomography (Phase 2)
- Progression-Free Survival (Phase 2/Phase 3)
- Prostatic Neoplasms (Phase 2)
- Protein Kinase Inhibitors (Phase 1/Phase 2)
- Respiratory Tract Diseases (Phase 1/Phase 2)
- Respiratory Tract Neoplasms (Phase 1/Phase 2)
- Skin Neoplasms (Phase 2)
- Small Cell Lung Carcinoma (Phase 3)
- Squamous Cell Carcinoma of Head and Neck (Phase 2)
- Stomach Neoplasms (Phase 2)
- Thoracic Neoplasms (Phase 1/Phase 2)
- Thyroid Neoplasms (Phase 2)
- Tumor Suppressor Protein p53 (Phase 2)
- Urinary Bladder Neoplasms (Phase 2)
- Uterine Cervical Neoplasms (Phase 2)
Clinical Trials:
A total of 145 trials were held for Osimertinib in four phases. One hundred thirty-three (133) organizations participated in these trials.
Side Effects:
Major side effects observed after the consumption of Osimertinib are:
- Diarrhea
- Dry Skin
- Sore Mouth
- Cough
Product CAS No
1421373-66-1
Therapeutic Action
Osimertinib is used as the first treatment for a certain type of nonsmall-cell lung cancer (NSCLC) that has spread to other parts of the body. It is also used in people who could not be treated successfully with other similar chemotherapy medications. Osimertinib is in a class of medications called kinase inhibitors.
Conclusion
Osimertinib is a drug that is used to treat chronic non-small-cell lung carcinomas. It has a molar mass of 499.62 g/mol and stability of more than two years of stability. Osimertinib is sold under the brand name Tagrisso. Besides treating cancer, there are many other experimental indications of Osimertinib, including lung disease, kidney neoplasm, carcinoma, breast neoplasm, etc. The most common side effects of Osimertinib are diarrhea, sore mouth, and cough.

