Velpatasvir copovidone is an antiviral orally available inhibitor drug that targets NS5A protein to treat HCV. This antiviral drug target the replication process of the non-structural protein 5A (NS5A) to stop the spread of hepatitis C in the human body.
Enquire Now
What is Velpatasvir-Copovidone?
Product Description:
Molar Mass: 883 g mol-1
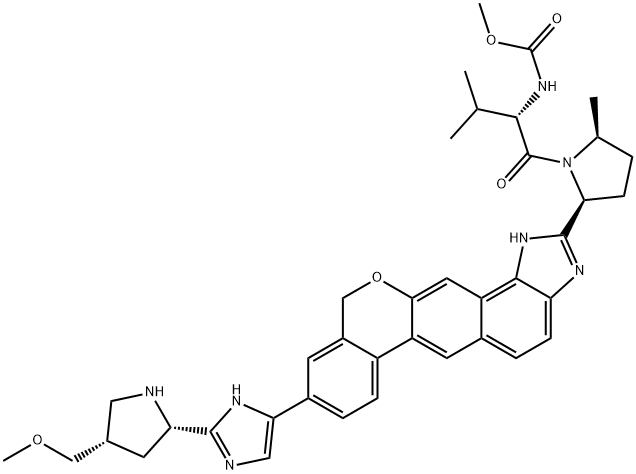
SMILES: COC[C@H]1C[C@H](N(C1)C(=O)[C@H](NC(=O)OC)C2=CC=CC=C2)C3=NC=C(N3)C4=CC5=C(C=C4)C6=CC7=C(C=C6OC5)C8=C(C=C7)N=C(N8)[C@@H]9CC[C@H](C)N9C(=O)[C@@H](NC(=O)OC)C(C)C
InChIKey: FHCUMDQMBHQXKK-CDIODLITSA-N
ALogP: 7.73
CAS Number: 152-11-4
Chemical Formula: C27H38N2O4•HCl
Therapeutic Category: AntiHSV
Form: Tablet
Assay: NLT 99.0%
Storage: Store at room temperature
Suitable for: Elderly, Adult
IUPAC Name:
methyl N-[(1R)-2-[(2S,4S)-2-[5-[6-[(2S,5S)-1-[(2S)-2-(methoxycarbonylamino)-3-methylbutanoyl]-5-methylpyrrolidin-2-yl]-21-oxa-5,7-diazapentacyclo[11.8.0.03,11.04,8.014,19]henicosa-1(13),2,4(8),5,9,11,14(19),15,17-nonaen-17-yl]-1H-imidazol-2-yl]-4-(methoxymethyl)pyrrolidin-1-yl]-2-oxo-1-phenylethyl]carbamate.
Approved Indication (2)
As per the clinical trials 1-4, Velpatasvir has two major approved indications:
- Hepatitis C
- Hepatitis C, Chronic
Experimental Indications (28)
- Bone Diseases, Metabolic (Phase 4)
- Buprenorphine (Phase 4)
- Carcinoma, Hepatocellular (Phase 4)
- Coinfection (Phase 4)
- Deglutition Disorders (Phase 1)
- Drug Misuse (Phase 4)
- End Stage Liver Disease (Phase 4)
- Heart Failure (Phase 2)
- Hepacivirus (Phase 1)
- Hepatitis B, Chronic (Phase 4)
- Hepatitis C (Phase 4)
- Hepatitis C, Chronic ()
- HIV (Phase 4)
- HIV Infections (Phase 4)
- Kidney Failure, Chronic (Phase 2)
- Kidney Transplantation (Phase 3)
- Liver Diseases (Phase 4)
- Liver Transplantation (Phase 2)
- Lung Diseases (Phase 1/Phase 2)
- Lung Transplantation (Phase 4)
- Methadone (Phase 4)
- Neoplasms (Phase 4)
- Opioid-Related Disorders (Phase 4)
- Organ Transplantation (Phase 4)
- Pharmacology (Phase 4)
- Pregnancy Complications (Phase 4)
- Respiratory Insufficiency (Phase 4)
- Thalassemia (Phase 4)
Clinical Trial
A total of 86 trials were held in four phases. 79 organizations took part in these trials.
Side Effects
Some common side effects seen in the patients taking Velpatasvir are:
- Headache
- Insomnia
- Nausea
- Fatigue
Conclusion
Velpatasvir is an antiviral drug generally used with Sofosbuvir to treat HCV. It belongs to NS5A inhibitors that restrict the replication of protein 5A. Velpatasvir copovidone has a molecular weight of 883 g mol-1 and a chemical composition of C49H54N8O8. You can take this medicine orally with a 100 gm per day dosage. Common side effects can include headache, fatigue, insomnia, and nausea.

