Table of Contents
What is Ibrutinib?
It is a type of medication known as a kinase inhibitor used to treat various blood cancers. It is also a complication of allogeneic stem cell transplants called chronic graft versus host disease. It helps to slow down the sudden progress of cancer by working against the cancerous B cells.
The mechanism starts by blocking Bruton’s Tyrosine Kinase signaling. A protein found in B cells instructs them to remain alive and multiply. Ibrutinib can also block the activity of a similar type of protein called Interleukin-2-Inducible T-cell Kinase.
API in Ibrutinib is also known as PCI-32765. It is crucial to keep in mind that the manufacturing procedure of Ibrutinib is quite complex, and it involves several steps for specific processes, there may be requirements for pharmaceutical raw materials.
The manufacturing process for Ibrutinib starts with the synthesis of API ingredients. API manufacturers play an important role in the production of Ibrutinib.
Ibrutinib was the first drug approved by the US food and drug administration in 2013.
Product Information
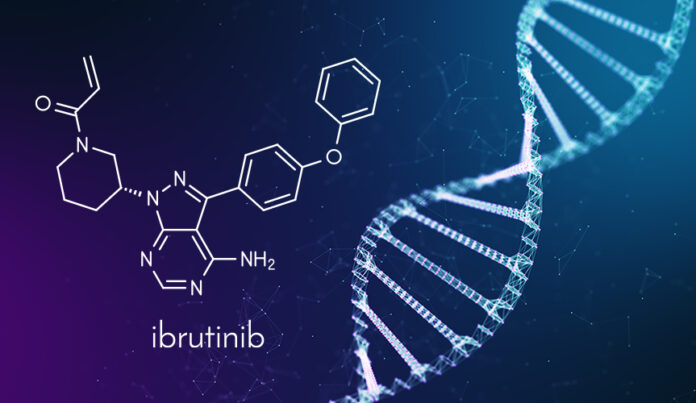
Chemical name of the Ibrutiunib is 1[(3R)-3-[4-amino-3-(4-phenoxyphenyl)-1Hpyrazolo[3,4-d]pyrimidin-1-yl]-1-piperidinyl]-2-propen-1-one.
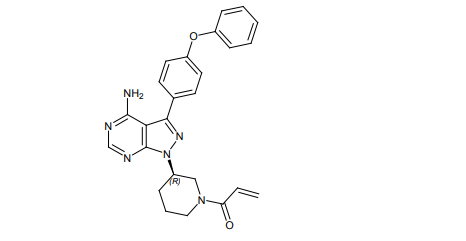
Molecular formula: C25H24N6O2
Molecular weight: 440.50
- It comes in the form of white capsules, which contain 140 mg of Ibrutinib as the active ingredient.
- The capsule’s Shell has gelatin, titanium dioxide, and black ink.
- At PH 1.2 Ibrutinib is slightly soluble whereas at PH 3 to 8 it is considered to be practically insoluble.
Mechanism of action
It is a small molecule inhibitor of Bruton’s Tyrosine Kinase. Ibrutinib creates a covalent bond in the active site of BTK with a cysteine residue. Bruton’s Tyrosine Kinase is a member of the Tec Kinase family, which is an important signaling molecule of the B cell.
Clinical Trials of Ibrutinib
The safety and efficacy of Ibrutinib have been on trial in Mantle Cell Lymphoma patients. One prior therapy was tested in the single open label. This drug was administrated orally at 560mg once on a daily basis until there is any disease progression or unacceptable toxicity.
Clinical Uses
FDA approves Ibrutinib for the treatment of several types of B cell malignancies, including
- Chronic Lymphocytic Leukemia
- Mantle cell lymphoma
- Waldenstorm macroglobulinemia.
Use of Ibrutinib
Ibrutinib is the medicine prescribed by doctors which are used to treat:
- Adults with Mantle cell Lymphoma who have already received at least one prior treatment.
- Adults who are diagnosed with Chronic Lymphocytic Leukemia / Small Lymphocytic Lymphoma.
- Adults who have Waldenstrom’s Macroglobulinemia.
- Children 1 year of age and adults with chronic graft versus host disease after the failure of 1 or more lines of systemic Therapy.
- Adults with marginal zone lymphoma require the medicine by injection or mouth and have received a specific type of prior treatment.
It is better if you consult an experienced physician before taking any drug. You must check the safety and effectiveness of the medicine as well.
Market Scenario
The demand for this drug suddenly increased due to the rise in the number of cancer patients worldwide and the increasing development of healthcare infrastructure regarding cancer. The increase in the number of blood cancer cases is the major contributing factor to the demand for Ibrutinib in the market.
Imbruvica (Ibrutinib) is thoroughly analysed in seven key markets, including the US and the EU5 countries, according to a report by Research and Markets. In a different research, DelveInsight examines Imbruvica’s sales statistics from 2017 through 2030 to assist clients in decision-making. Imbruvica’s manufacturer, AbbVie, has filed 165 patent applications for the medication.
The Lugano 2014 criteria may possibly boost response rates because patients in trials of acalabrutinib and zanubrutinib were exposed to these drugs sooner in the course of treatment than they were with ibrutinib. Patients with MCL who did not get a complete response (CR) after first-line treatment have a significantly higher mortality rate and greater drug resistance. Ibrutinib, a single drug, had a 20% rate of CR in MCL throughout long-term follow-up, although its 2-year PFS and OS rates were 79% and 92%, respectively.
Due to substantial research into the treatment of the indicated condition and increased healthcare spending globally, the market for Ibrutinib appears to have a bright future. This will allow medication producers to extend their market share. Due to the growing number of cancer patients worldwide and the expansion of the infrastructure for treating cancer, Ibrutinib demand is anticipated to rise. Leukemia, Lymphoma, and Myeloma are just a few of the blood cancers that can be treated with ibrutinib. Ibrutinib has additionally demonstrated promise in the management of solid tumors. The drug producers are utilising a variety of techniques as the market environment for ibrutinib is expected to alter in the upcoming years.
Side Effects of Ibrutinib
Like any other drug Ibrutibib can have serious side effects on your body, including:
- Bleeding problems are quite common during the treatment with Ibrutinib and can be serious and may lead to death. The bleeding risk can increase if you take a blood thinning medicine. You have to consult the healthcare providers right away in case you have any signs of bleeding, which may include:
- Blood in the stool or black stool;
- Brown or pink urine
- Unexpected bleeding.
- Vomit looks like coffee grounds or blood vomit
- Bruising
- Dizziness
- Confusion
- Weakness
- Heachache, which lasts for a long time, or a severe headache.
- Muscle and bone pain
- Fatigue
- Diarrhea
- Infections may happen during the treatment with Ibrutinib. All these infections can be really serious and may have fatal consequences. Consult your healthcare provider beforehand if you have a fever, chills, weakness, or any other symptoms of infection during the treatment with Ibrutinib.
- It was observed people who have been treated with Ibrutinib might experience high blood pressure. Your healthcare provider may start with the blood pressure medicine or change the current one to treat the blood pressure first.
- Decrease in the blood cell counts are common with Ibrutinib, but that can also be severe. Your doctor should do monthly checkups and blood tests to check the blood count.
Apart from this, other common side effects of Ibrutinib in adults or children one year of age and old may experience:
- Fatigue
- Joint and muscle pain
- Fever
- Stomach pain
- Muscle Spasms
- Pneumonia
- Mouth Sores
- Headache
- Bruising
- Diarrhea
Diarrhea is one of the common side effects in people who consume Ibrutinib. Drinking plenty of fluids during the treatment can reduce the potential risk of dehydration due to diarrhea.
- People treated with ibrutinib may experience serious heart problems and heart failure. It increases the risk of heart disease. If you experience any symptoms of heart problems, such as fast and irregular heartbeat, shortness of breath, dizziness, or lightheadedness, then immediately consult a healthcare professional.
Precautions To Be Observed With Ibrutinib
Before taking Ibrutinib, consult the healthcare professional about the medical conditions in case you:
- If you have had any recent surgery or are planning to have surgery, the healthcare service provider should stop giving you ibrutinib for any planned surgical, medical, or dental procedure.
- Having bleeding issues.
- Heart rhythm problems or any medical condition which increases the risk of heart diseases, such as high blood pressure, high cholesterol, or diabetes.
- Have an infection.
- Have liver problems.
Conclusion
Ibrutinib is clinically tested for the treatment of certain kinds of cancers, such as chronic lymphocytic leukemia. We covered the usage, side effects, and dose information. For more knowledge of the drugs and their composition, you can always contact Bulat Pharmaceutical. We are here to provide you with all the information related to the drugs.